Seen in New Zealand
/Islands seen from another island:
Plants and animals seen in city parks:
Views seen from boats, train, and helicopter:
Native birds (and kererū feather) seen:
Things seen from shore:

Artwork for Oikos journal, illustrating the study Sources of confusion in global biodiversity trends by Boënnec et al.
A fog-filled session of retrieving tags from Leach’s Storm-Petrels (and eating wild strawberries) on Little Duck Island, which is (usually) uninhabited by humans.













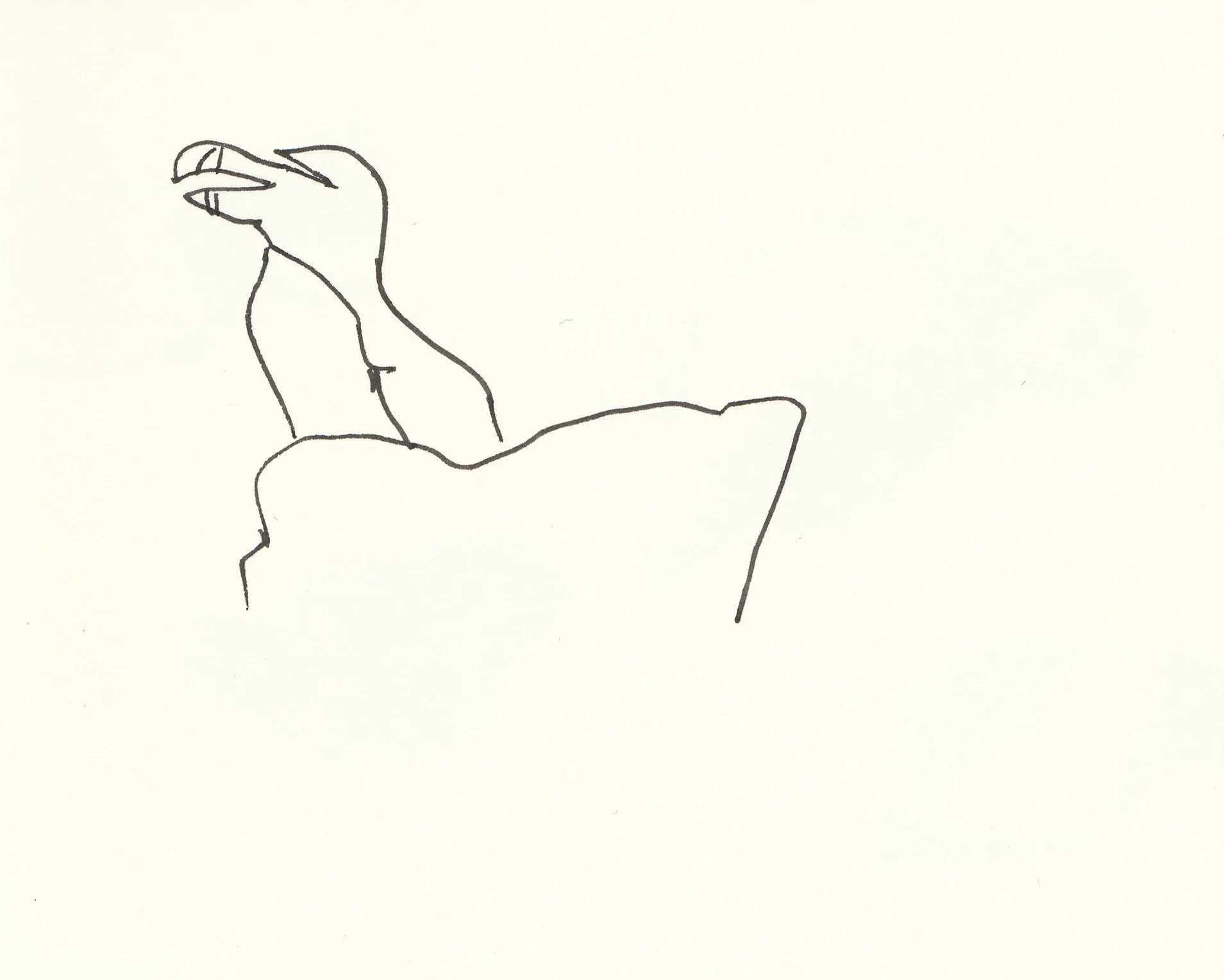
Razorbill (Alca torda) on Machias Seal Island, Maine Canada Atlantic Ocean
Abby McBride
© Abby McBride
abbymcb@alum.mit.edu
Top photo credit: Otto Whitehead